If you are a non technical buyer we thought it might be useful if we gave you a quick overview of how fuel cells actually work.
We have deliberately tried to keep it simple, but if you want more detail please give us a call and we will be happy to answer your questions.
Definitions
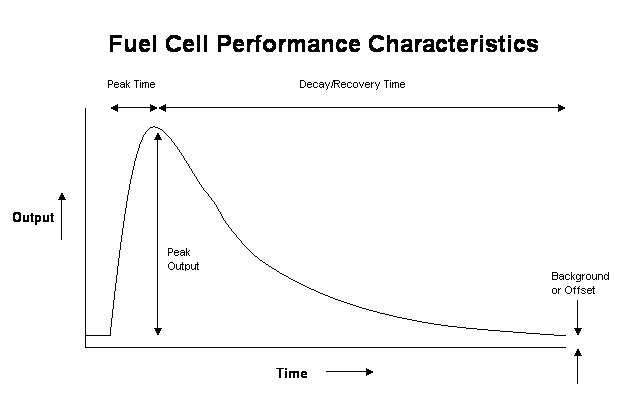
To be able to discuss fuel cells we first need to be sure we are both talking about the same things. We use the following terms to describe the fuel cell performance characteristics:
- Peak Output – The maximum voltage or current generated from the cell after a sample has been introduced.
- Peak Time – The time taken to reach the Peak Output above.
- Background/offset – The steady state voltage or current output from the cell prior to introduction of the sample.
- Decay/clearing Time – The time taken for the cell to return to the steady state offset.
- Repeatability – The precision of the Peak Output achieved from successive identical samples.
- Calibration Stability – The precision of the Peak Output achieved with samples taken over a long period of time.
- Dead Space – The volume of air in the system that does not move until a sample is taken.
- Linearity – The correlation precision of the Peak Output at increasing gas concentrations.
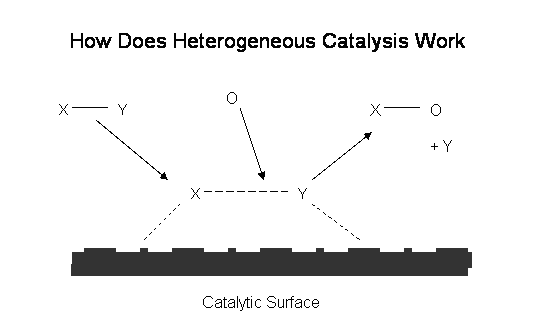
Basic Scientific Principles
In simple terms, we use a catalyst to convert the ethanol molecule into a new molecule ethanoic acid which alters the electrical properties of the electrode. This alteration creates the peak output from the fuel cell. For those of you who want a little bit more science this process is known as Heterogeneous Catalysis.
Heterogeneous catalysis works by reducing the energy (usually in the form of heat) needed for a given reaction to take place. This works by the catalyst having an affinity (attraction) for the molecule to be reacted, in this case X-Y and thus stretching and weakening the bond between the two molecules. The position on the catalyst where this occurs is known as an “active site” and is on an intermolecular scale, measured in nanometres. While the bond is weakened in this way it takes less energy to break it down and form new bonds with other reactant molecules, shown as O above. This yields a new molecule X-O above and respective by-products at a lower required energy (temperature). In the case of the reduction of ethanol to ethanoic acid this would occur at normal ambient temperatures in presence of a suitable catalyst. At the end of such a reaction the catalyst is unchanged both chemically and physically and is subsequently reusable.